TAKE HOME MESSAGES :
- The ENS orchestrates gut motility through a complex balance of neural circuits and neurotransmitters.
- Disruptions in gut motility can trigger systemic metabolic imbalances, contributing to hyperglycemia and insulin resistance.
- ENS dysfunction is now recognized as a key player in the pathophysiology of metabolic syndrome.
- Understanding and targeting gut motility opens up new therapeutic perspectives for metabolic disorders.
Did you know that your gut is in constant motion, even when you’re at rest?
Every segment of your intestine follows a meticulously coordinated pattern of contractions and relaxations to propel food, absorb nutrients, and eliminate waste. But what orchestrates this seamless choreography? The Enteric Nervous System (ENS)—our “second brain”—ensures the precise regulation of gut motility through a complex interplay of neurons, neurotransmitters, and local reflex circuits.
What is gut motility ?
Gut motility is a fundamental component of digestion. These rhythmic contractions, essential for maintaining digestive homeostasis, are regulated by a complex interplay of neural, muscular, and biochemical factors.
When gut motility is disrupted, a wide range of gastrointestinal disorders can arise, including constipation, diarrhea and irritable bowel syndrome (IBS). These conditions impact not only digestive health but also overall well-being, as inefficient gut transit can lead to malabsorption, inflammation, and altered metabolic function.
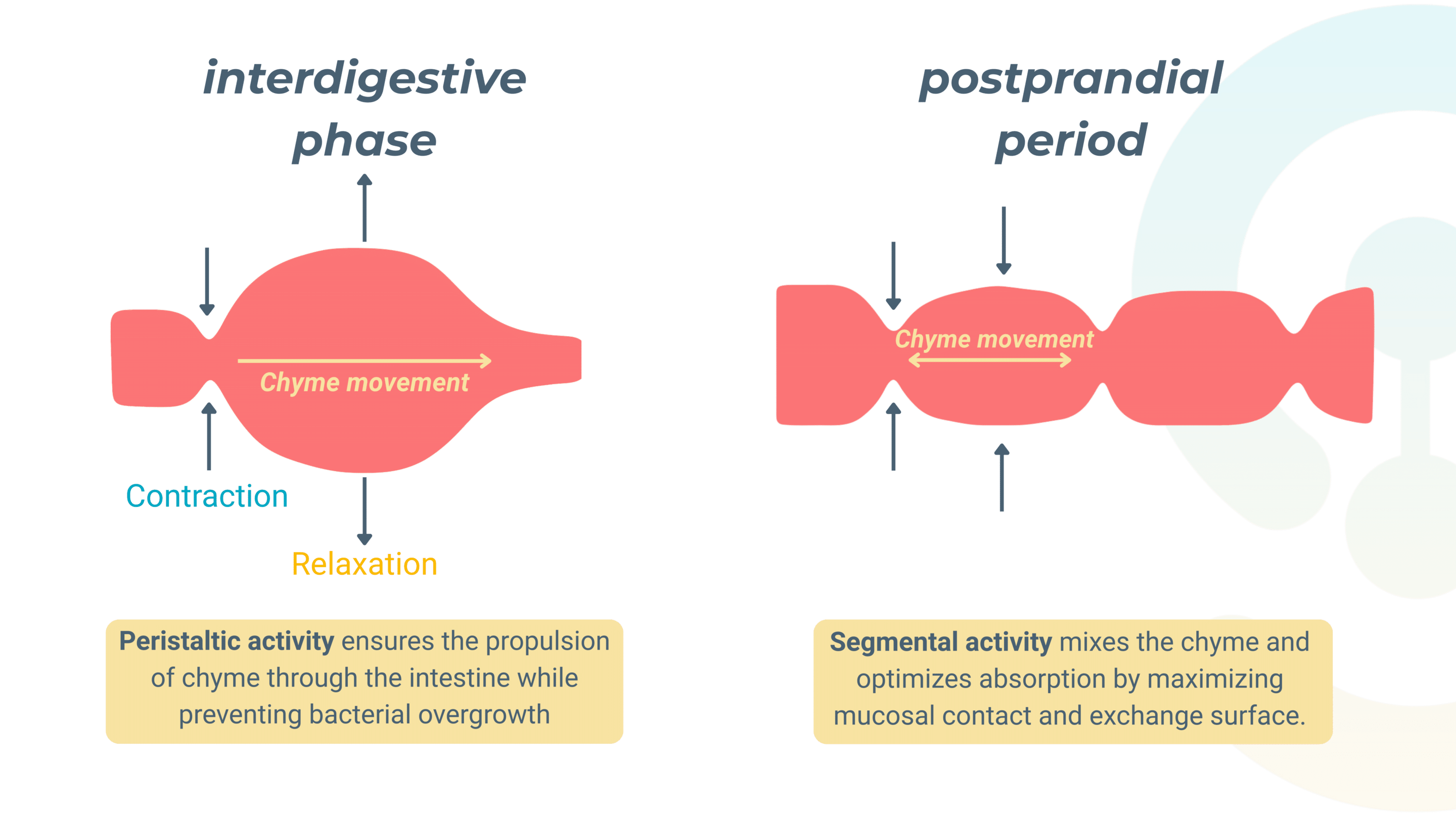
The gut motility regulated by the ENS
Functional structure of the ENS
The ENS is unique among neural systems because it functions autonomously while maintaining communication with the CNS. It is structured into two primary neural plexuses that work together to regulate gut motility:
The myenteric plexus (Auerbach’s plexus)
Positioned between the circular and longitudinal muscle layers, this network controls the strength, speed, and coordination of intestinal contractions, ensuring the propulsion of food through the digestive tract.
The Submucosal plexus (Meissner’s plexus)
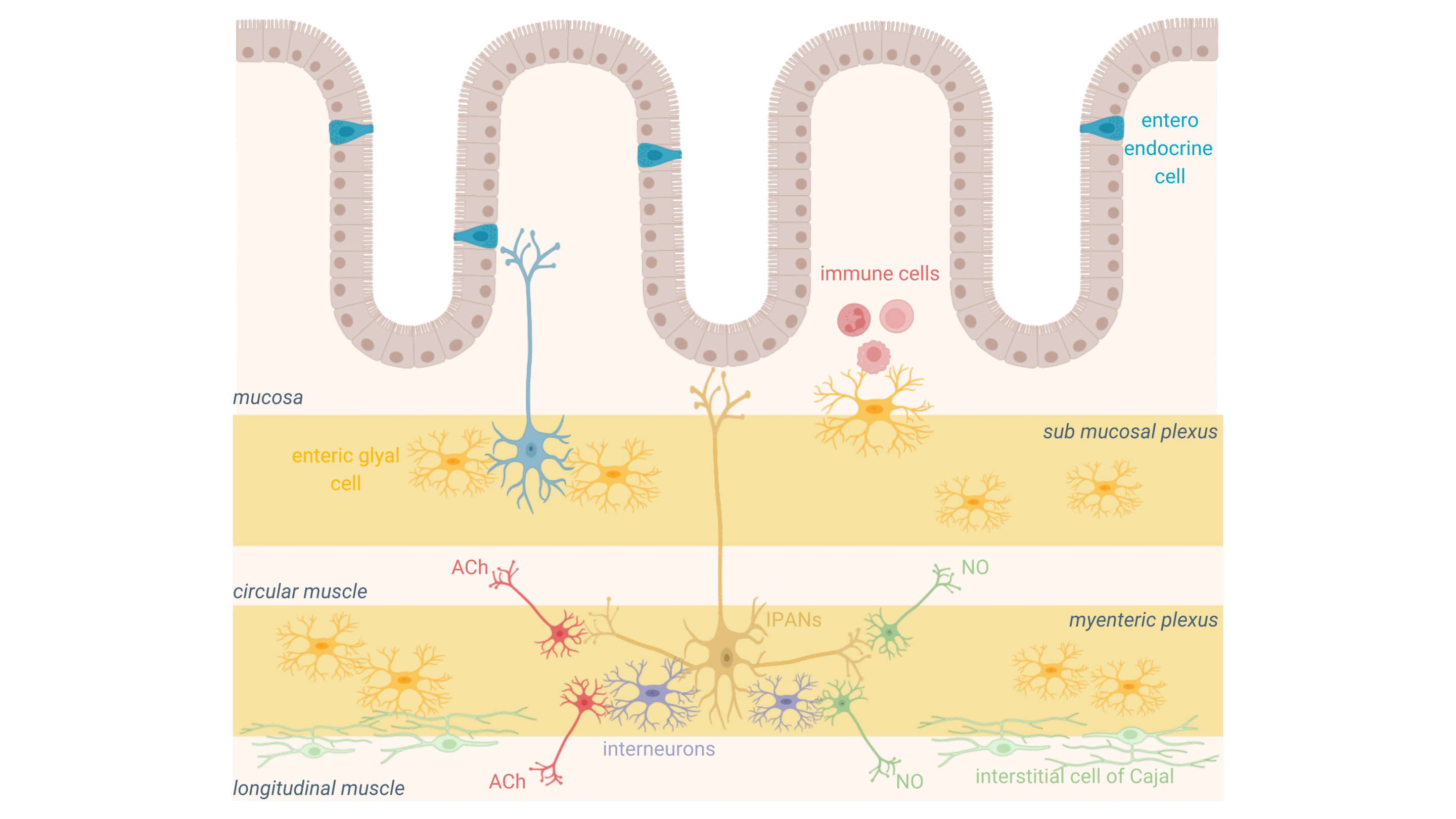
These two plexuses interact dynamically, integrating sensory and motor responses to ensure efficient and adaptive gut transit.
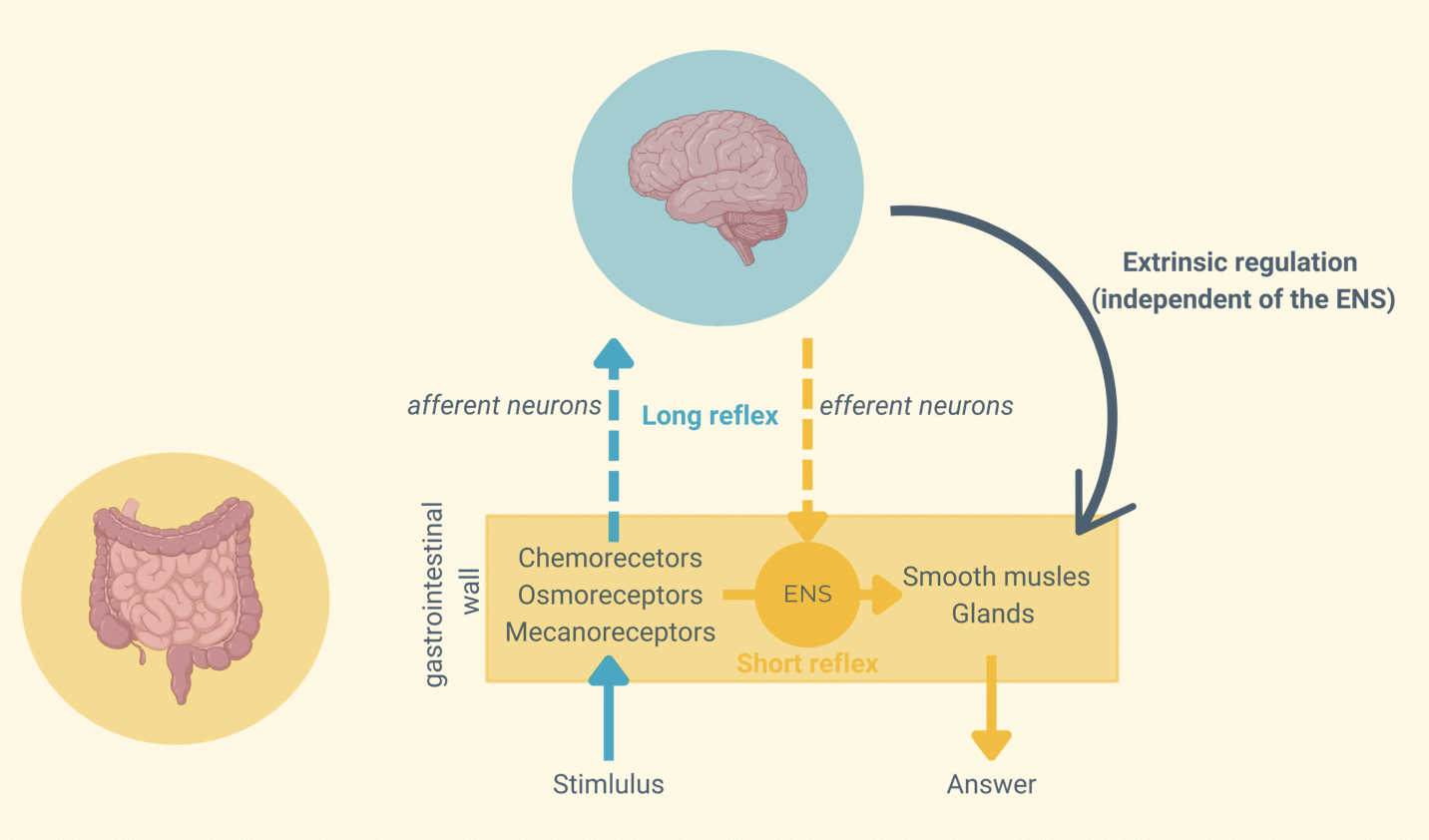
Gut motility: a dual regulation by intrinsic and extrinsic loops
Gut motility is governed by two interconnected regulatory pathways—an intrinsic loop controlled by the ENS and an extrinsic loop modulated by the CNS. Together, these mechanisms allow the gut to self-regulate digestion while responding to external physiological demands.
The Intrinsic Loop (Short Reflexes)
This ENS-driven mechanism functions entirely within the gut wall, allowing local control of motility.
Sensory receptors—chemoreceptors, osmoreceptors, and mechanoreceptors—detect changes in nutrient composition, osmolarity, and mechanical stretch, sending signals to motor neurons within the ENS.
These neurons then trigger immediate responses that regulate smooth muscle contractions and glandular secretions, ensuring the gut adapts to its local environment without requiring CNS input.
The Extrinsic Loop (Long Reflexes)
Neurotransmitter balance: the core of ENS regulation in gut motility
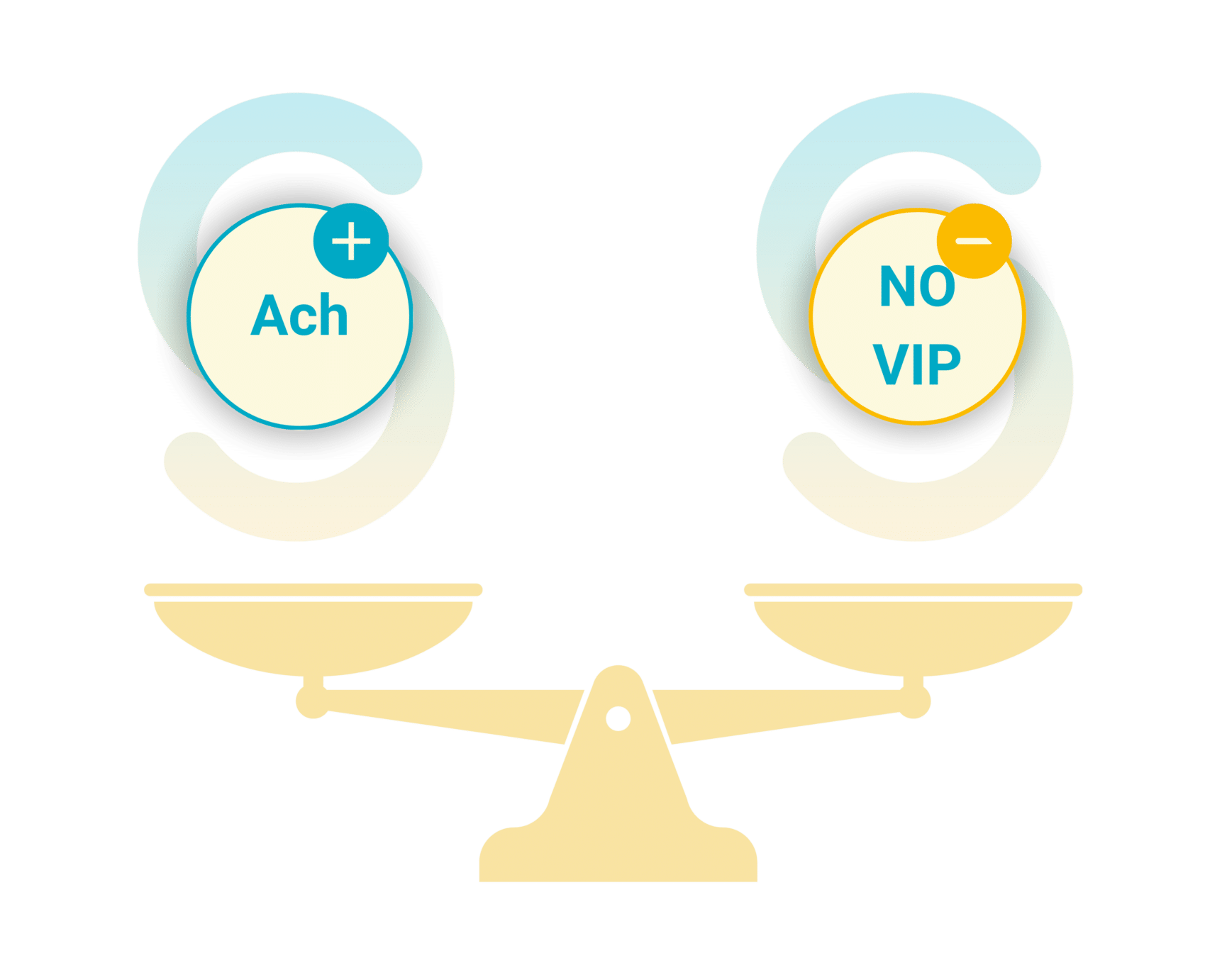
At the center of ENS regulation is a fine balance of neurotransmitters, which coordinate motility patterns to ensure smooth digestive transit. The most critical neurotransmitters include:
- Acetylcholine (ACh)
As the primary excitatory neurotransmitter, ACh is essential for stimulating intestinal contractions. It activates cholinergic motor neurons, which trigger smooth muscle contraction, facilitating the forward propulsion of food through the gut.
- Nitric Oxide (NO) and Vasoactive Intestinal Peptide (VIP)
These inhibitory neurotransmitters work in opposition to ACh by inducing muscle relaxation. They prevent excessive contractions, ensuring a coordinated and controlled propulsion that avoids spasms and blockages.
- Serotonin (5-HT)
Secreted primarily by enterochromaffin cells in the gut lining, serotonin is a key modulator of peristalsis. It acts on intrinsic primary afferent neurons (IPANs), triggering motility reflexes and fine-tuning gut sensitivity. Imbalances in serotonin signaling are strongly associated with gut motility disorders, including IBS and functional dyspepsia.
Understanding the interplay between ENS circuits, neurotransmitters, and physiological modulators (such as food, microbiota…) opens the door to new therapeutic strategies targeting gut motility to prevent or cure systemic disorders.
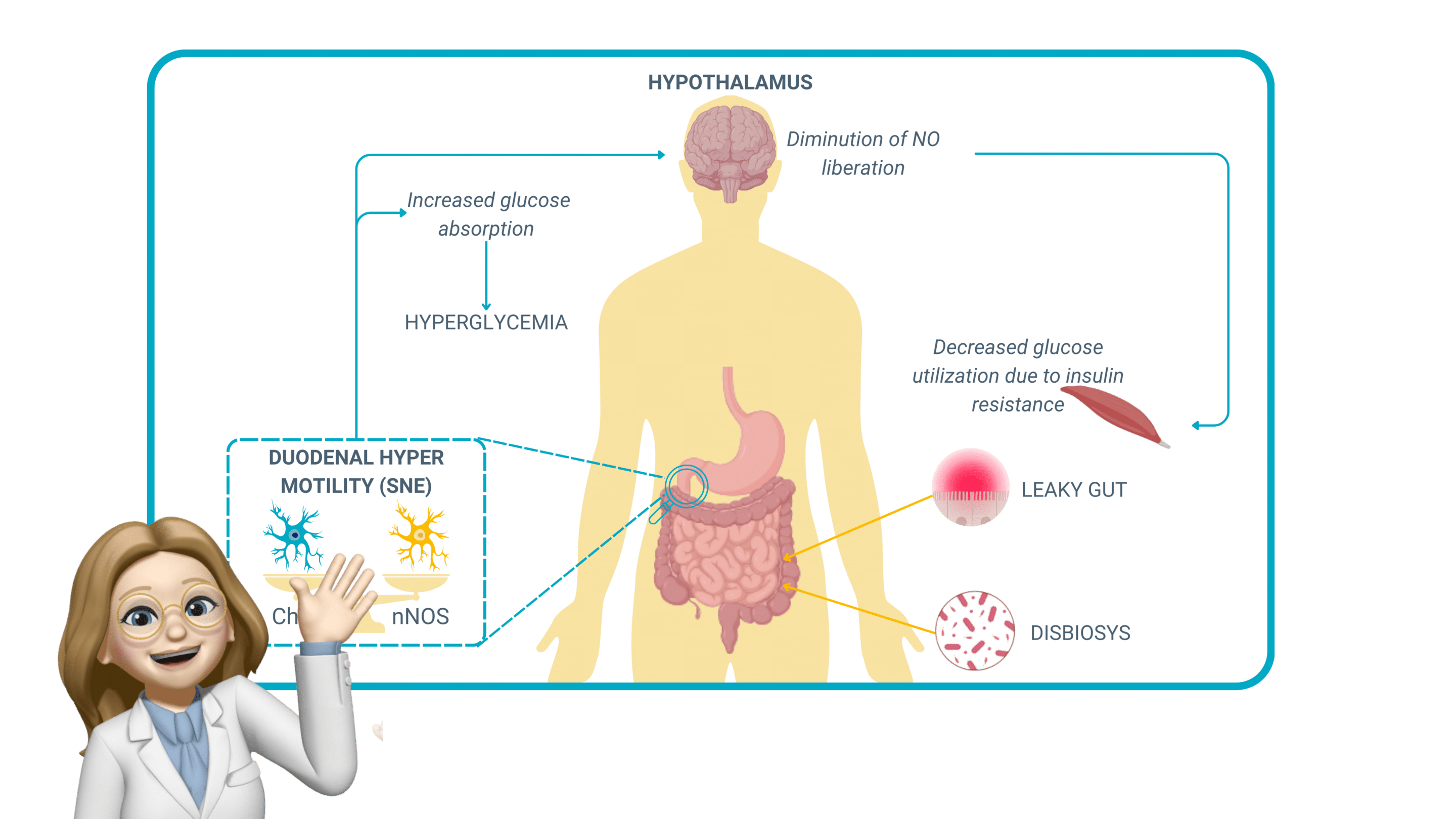
Pathologies and dysfunctions of ENS-regulated gut motility
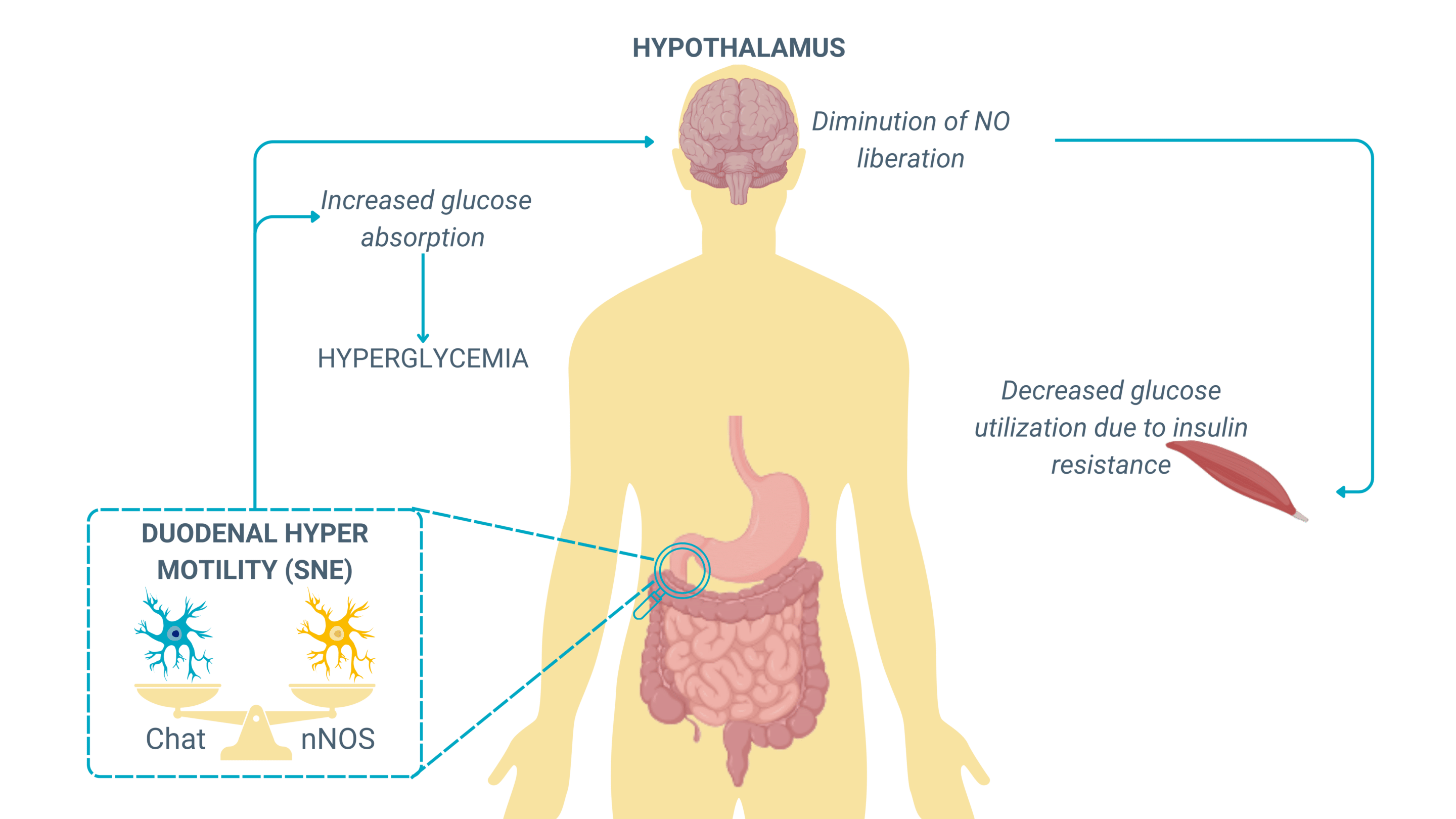
Absorption of glucose due to excessive motility
An increase in gut motility can paradoxically lead to an increased glucose absorption, a mechanism that plays a crucial role in metabolic diseases. When motility is too rapid, luminal contents move too quickly through the small intestine, but instead of reducing absorption, this upregulates glucose transporters (SGLT1 and GLUT2) in the intestinal epithelium as a compensatory mechanism.
This phenomenon is observed in early-stage metabolic disorders and obesity, where:
- Hyperactive motility increases glucose transporter expression, leading to higher-than-normal glucose absorption rates, even when dietary intake is not excessive.
- Persistent glucose overload contributes to hyperglycemia, increasing insulin demand and fostering insulin resistance.
- The ENS, influenced by vagal efferent signaling, further reinforces gut-brain metabolic loops, exacerbating metabolic dysregulation.
This mechanism suggests that gut motility dysfunctions may precede and contribute to metabolic disorders such as type 2 diabetes (T2D) by promoting early hyperinsulinemia and systemic glucose dysregulation.
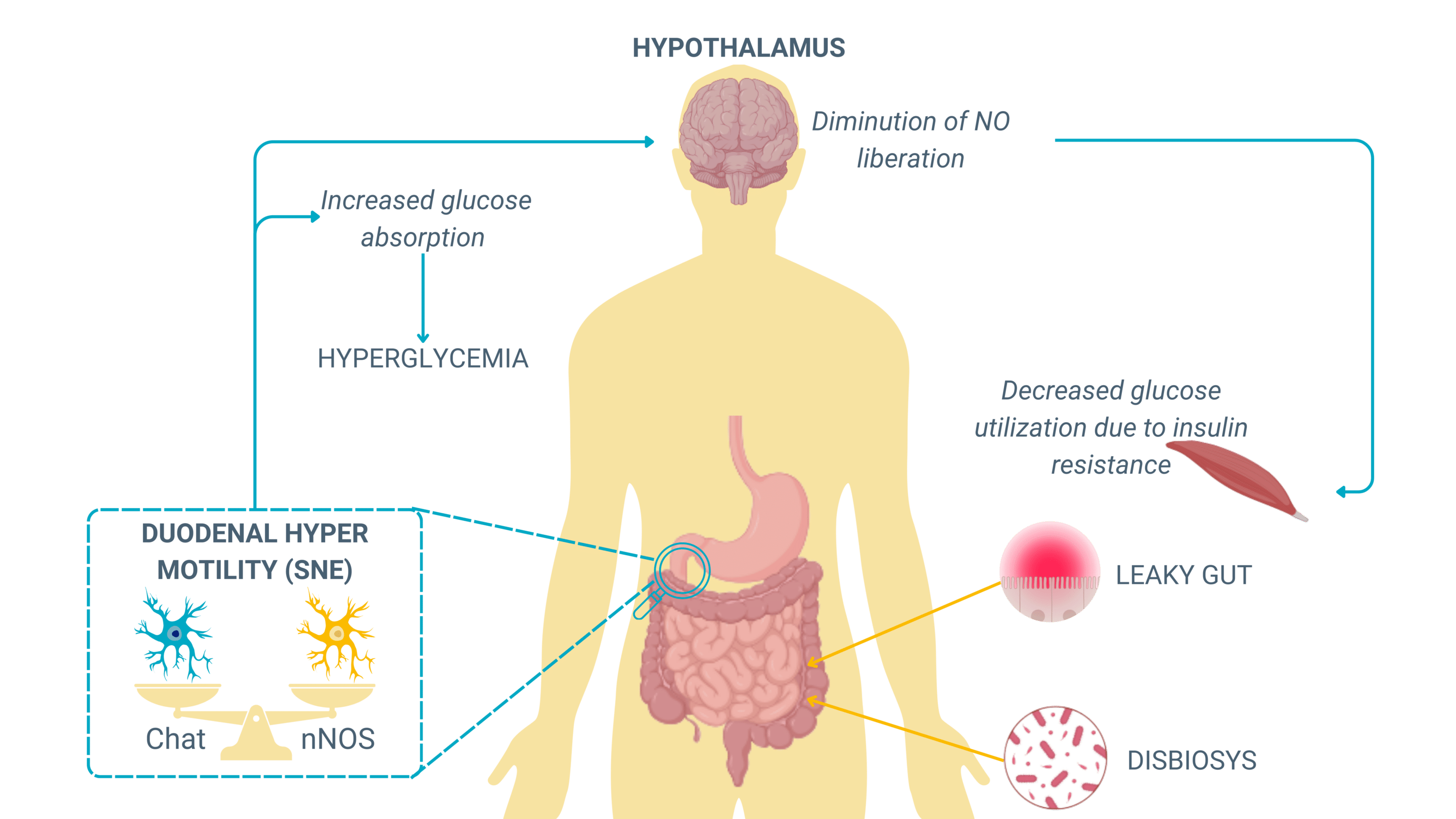
Chronic ENS dysregulation and metabolic syndrome
Chronic alterations in ENS function contribute to metabolic syndrome, an interconnected set of conditions—including obesity, hyperglycemia, insulin resistance, and dyslipidemia—that collectively increase the risk of developing metabolic diseases.
ENS dysfunction in this context manifests through:
- Disrupted enteric neuron signaling, affecting the balance between excitatory (ACh-mediated) and inhibitory (NO-mediated) motility regulation.
- Increased oxidative stress and inflammation, promoting leaky gut and neuronal damage and impairing neuromuscular coordination in the gut.
- Altered microbiota composition, which influences ENS excitability and further dysregulates intestinal transit.
Patients with metabolic syndrome often present with erratic bowel movements, alternating between accelerated motility (leading to glucose hyperabsorption) and disturbed gastric emptying, creating fluctuating blood glucose levels that worsen insulin resistance.
Far from being a simple digestive assistant, the Enteric Nervous System (ENS) plays a central role in coordinating gut motility and, by extension, systemic metabolic balance.
When this intricate regulation is disrupted, the consequences go far beyond the gut—impacting glucose absorption, insulin sensitivity, and contributing to the onset of metabolic syndrome. These insights pave the way for innovative therapeutic approaches that target the ENS and intestinal motility as potential levers to prevent or reverse metabolic diseases.
Stay tuned! In the next issue, we’ll dive into the most promising therapies and research strategies aimed at modulating the ENS and restoring motility balance—offering new hope not only for treating but also for preventing metabolic disorders such as obesity, type 2 diabetes, and insulin resistance!
To craft this newsletter, we drew insights from the work of Claude Knauf.