Pharmaceutical testing: accelerating drug development with tailored solutions
In today’s fast-evolving pharmaceutical landscape, the demand for efficient and reliable pharmaceutical testing has never been greater. At Enterosys, we specialize in providing comprehensive preclinical testing solutions.
What is pharmaceutical testing? Understanding pharmaceutical drug identification
Pharmaceutical testing plays a vital role in modern healthcare by ensuring that only safe, effective, and high-quality drugs reach the market.
It encompasses a series of rigorous evaluations designed to identify and validate pharmaceutical drugs. This process includes the analysis of active pharmaceutical compounds, molecular structures, availability and other key components to determine their therapeutic potential.
At its core, pharmaceutical drug identification involves pinpointing the unique characteristics of a compound, ensuring it meets the intended therapeutic goals.
But what exactly does “pharmaceuticals” mean? Pharmaceuticals refer to any substance used to prevent, diagnose, treat, or relieve symptoms of a disease. Their development requires careful testing to ensure both efficacy and safety.

Challenges in the pharmaceutical industry: developing complex drugs for complex diseases
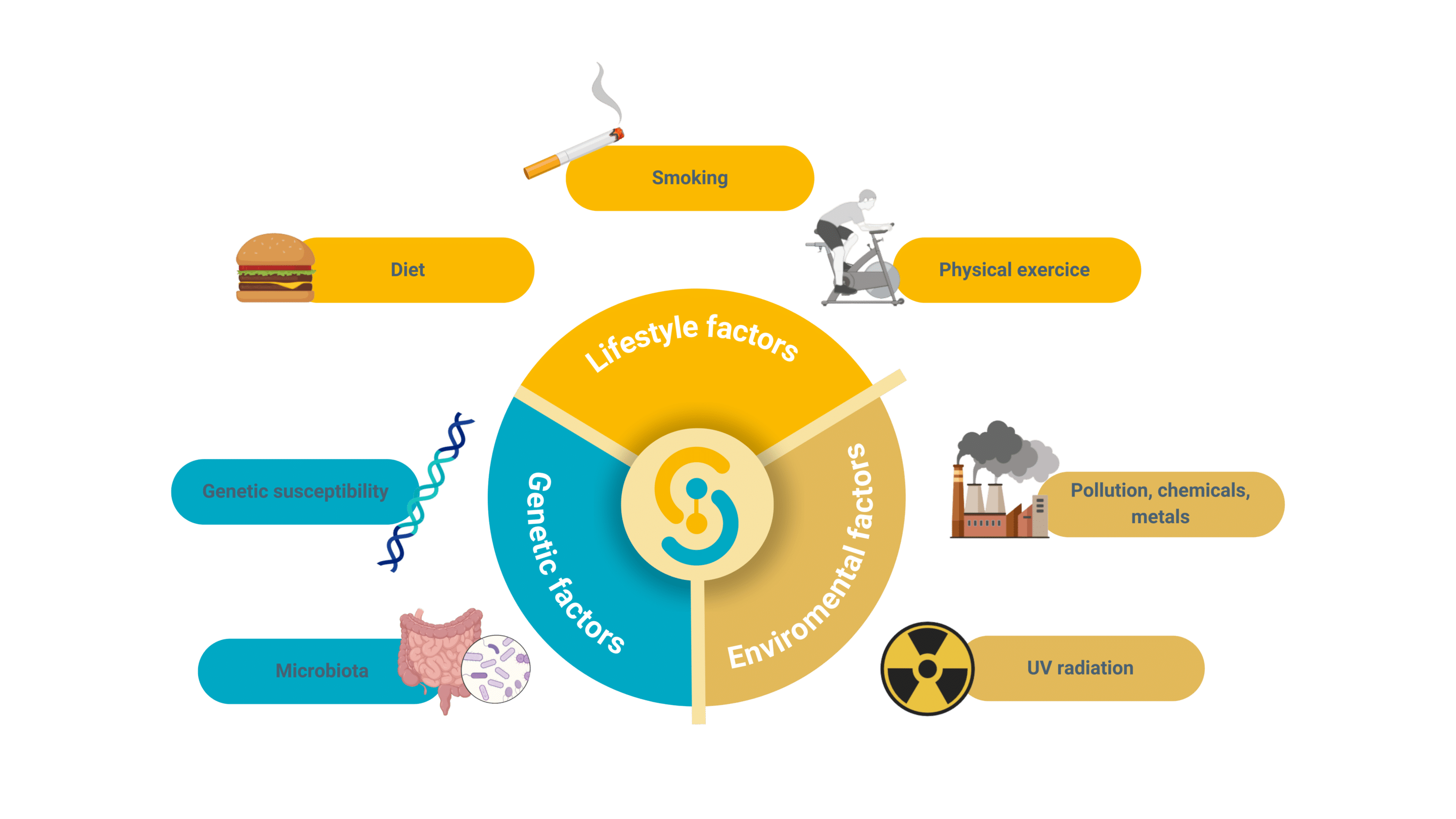
The pharmaceutical industry faces significant challenges in developing treatments for complex, multifactorial diseases. These conditions, such as autoimmune disorders and systemic diseases, result from intricate interactions between genetic, environmental, and lifestyle factors. Their complexity makes the development of effective therapies particularly demanding.
Multifactorial diseases, such as type metabolic, neurodegenerative, dermatologic and gastrointestinal disorders, arise from a combination of genetic predispositions and environmental influences.
The development of drugs targeting these complex diseases requires a deep understanding of their multifaceted nature. Personalized medicine approaches are increasingly important, as they allow for treatments tailored to the unique genetic and environmental profiles of individual patients. This strategy is essential for addressing the variability in disease manifestation and treatment response observed in systemic and multifactorial diseases.
Why preclinical pharmaceutical testing is critical for drug development
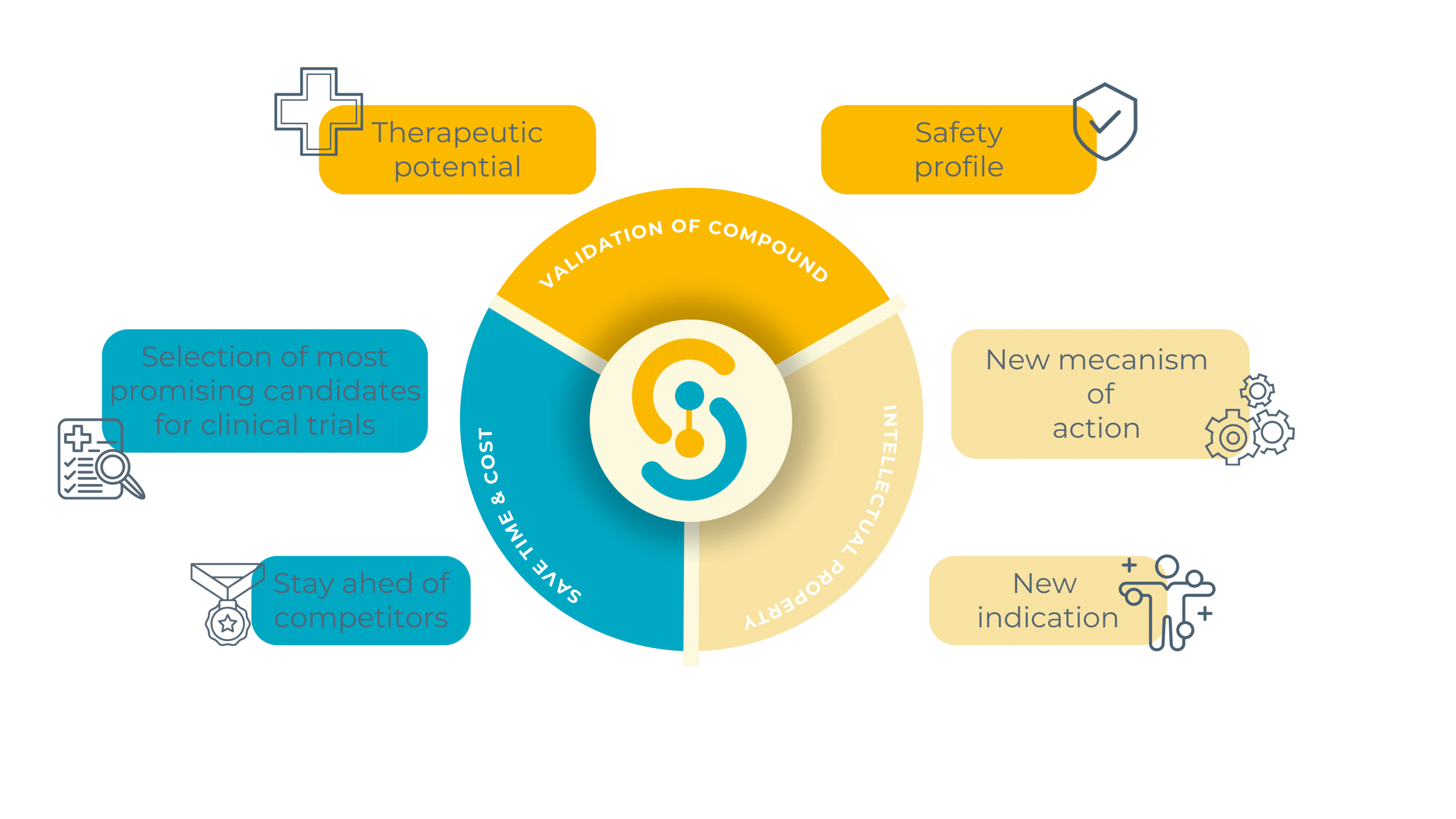
Before any drug reaches human trials, it must undergo preclinical testing. These tests validate a compound’s safety profile and therapeutic potential, ensuring only the most promising candidates proceed to clinical trials. Preclinical pharmaceutical testing saves time, reduces costs, and helps avoid potential failures in later stages, protecting both patients and investors.
Speeding up pharmaceutical drug identification in a competitive landscape
In today’s fast-paced pharmaceutical industry, speed is everything. Rapid pharmaceutical drug identification allows companies to stay ahead of competitors. This process involves comparing new drug candidates with existing treatments to evaluate their therapeutic effects and mechanisms of action. In some cases, it even enables the repositioning of known compounds for new uses, accelerating development timelines. By redirecting a known compound to a new indication or uncovering a novel mechanism of action, companies can file new patents, creating additional intellectual property. This generates added value through exclusive rights to use the compound in this specific context, offering a significant competitive advantage.
Enterosys’ pharmaceutical drug testing offerings
Custom in vitro and in vivo models for pharmaceutical drug testing

On the other hand, we personalize each project by working closely with our clients to design experiments that address their specific research questions. Whether it’s characterizing a mechanism of action or assessing the non-regulatory toxicity of a compound, our bespoke approach delivers reliable, actionable data.
We understand that the emergence of complex diseases and the growing demand for personalized treatments require preclinical testing results to be highly translatable. To meet this challenge, Enterosys offers bespoke in vitro and in vivo models that not only simulate real-world conditions but also account for the specific phenotypes of the targeted pathologies. These advanced models are essential for accurately assessing a drug’s efficacy and safety across diverse disease states, providing researchers with reliable and clinically relevant data before advancing to human trials.
Why Gut is the link ?
Hippocrates, the father of modern medicine, famously stated over 2,000 years ago:
“All disease begins in the gut.”
While this may have been seen as revolutionary in ancient times, modern science is proving just how visionary his perspective truly was.
Today, the gut is recognized not only as a key regulator of health but also as a central hub for interactions that shape systemic diseases, from metabolic disorders to neurodegenerative conditions.
This gut-centered approach not only provides a more holistic understanding of the body but also unveils therapeutic opportunities that would otherwise remain overlooked, ensuring a deeper, more accurate evaluation of potential treatments.
Why choose enterosys for pharmaceutical testing?
At Enterosys, we adhere strictly to pharmaceutical guidelines that guarantee the quality, reliability, and safety of our preclinical research, offering our clients confidence in the data generated.
In addition to compliance, Enterosys is accredited under the Crédit d’Impôt Recherche (CIR), a French government initiative designed to support companies engaged in R&D activities. This accreditation is a mark of excellence, recognizing Enterosys as a trusted research partner. Being CIR-accredited means that our clients benefit not only from rigorous scientific methodologies but also from potential financial incentives, making high-quality research both accessible and cost-effective.
Enterosys combines speed, precision, and customization to offer tailored solutions that address the unique challenges of each project! Contact Enterosys now to learn how we can support your pharmaceutical testing needs!

White paper ENTEROSYS
« GUT IS THE LINK »
Our first white paper is now available !
Get inside the head of our co-founder Claude Knauf.
Come discover his background, his key dates, how he got the gut feeling and the link between enteric neurons and glycemic control as well as other health related areas.

OUR KEY SCIENTIFIC FINDINGS ABOUT THE GUT
The gut-brain axis: exploring its role in health care
In the intestine, gut distension and nutrients are detected by mechanoreceptors and chemoreceptors, respectively. The activation of these receptors sends an afferent nervous message to the hypothalamus in the brain. In turn, the hypothalamus controls the glucose entry in tissues, and thus glycemia.
12-HETE enterosyne: new insights into glucose metabolism
The discovery of intestinal actors, such as enterosynes, able to modulate the ENS-induced duodenal contraction is an innovative approach. Among all the intestinal factors, the understanding of the role of gut microbes in controlling glycaemia remains a major target. For instance, we researched and demonstrated how the modulation of gut microbiota by prebiotics could permit the identification of novel enterosynes.
Glucose: a key player in gut motility and metabolic regulation
Targeting the enteric nervous system that controls gut motility is now considered as an innovative therapeutic way in T2D to limit intestinal glucose absorption and restore the gut‐brain axis to improve insulin sensitivity. So far, little is known about the role of glucose on duodenal contraction in fasted and fed states in normal and diabetic conditions.
Do you have a question about the contribution of gut models in your innovative research ?
Our team of experts will be delighted to answer all of your questions. We guide you in the design of an optimized protocol to meet your objectives and add value to your molecules with quick and concrete solutions.