Did you know that the enteric nervous system, often referred to as our “second brain,” is composed of over 200 million neurons lining the digestive tract?
It plays a critical role in maintaining digestive health and mediating the connection between the gut and the brain. Its influence extends beyond digestion, impacting mood, metabolism, and overall well-being through the gut-brain axis.
What is the enteric nervous system?
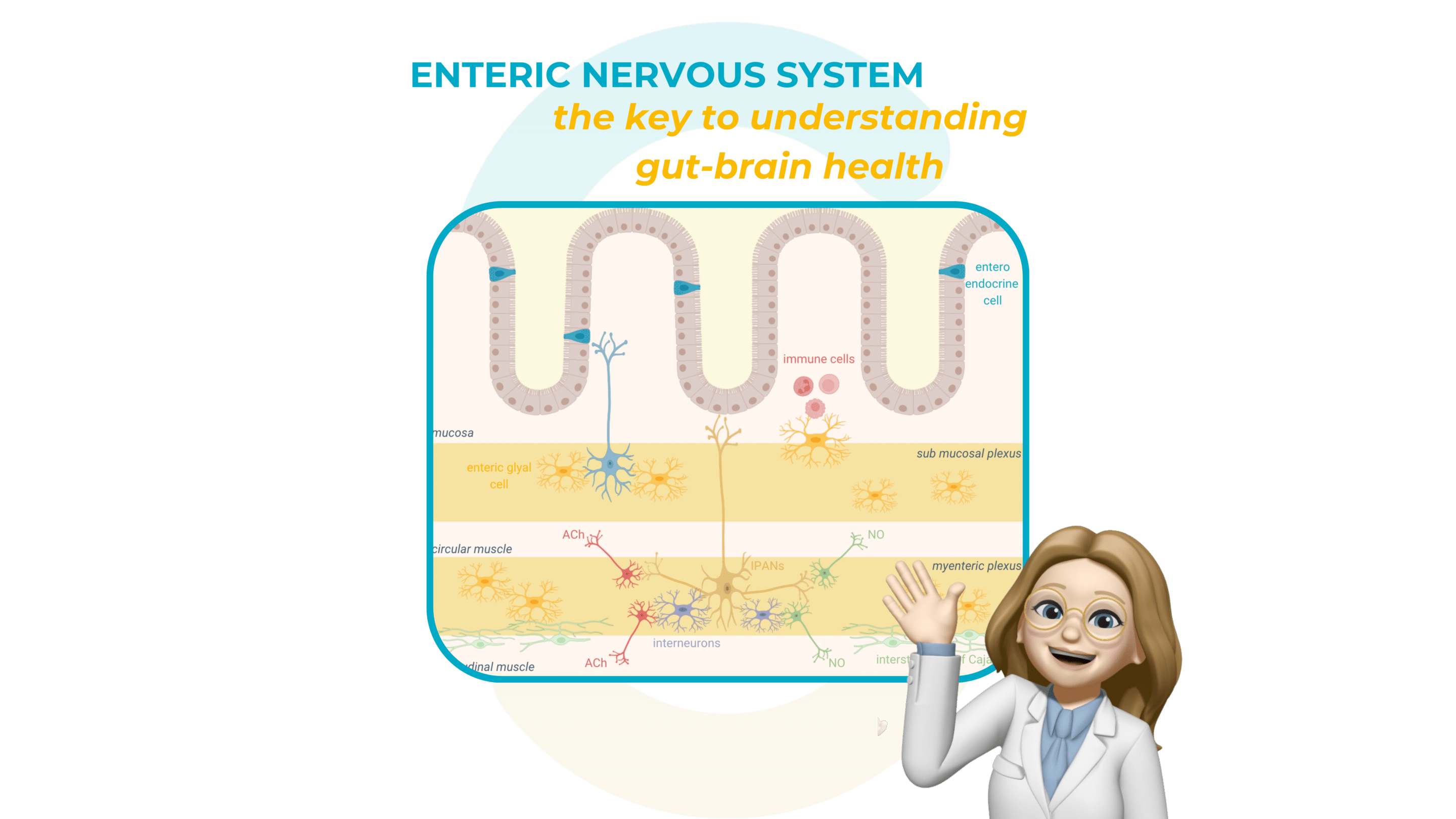
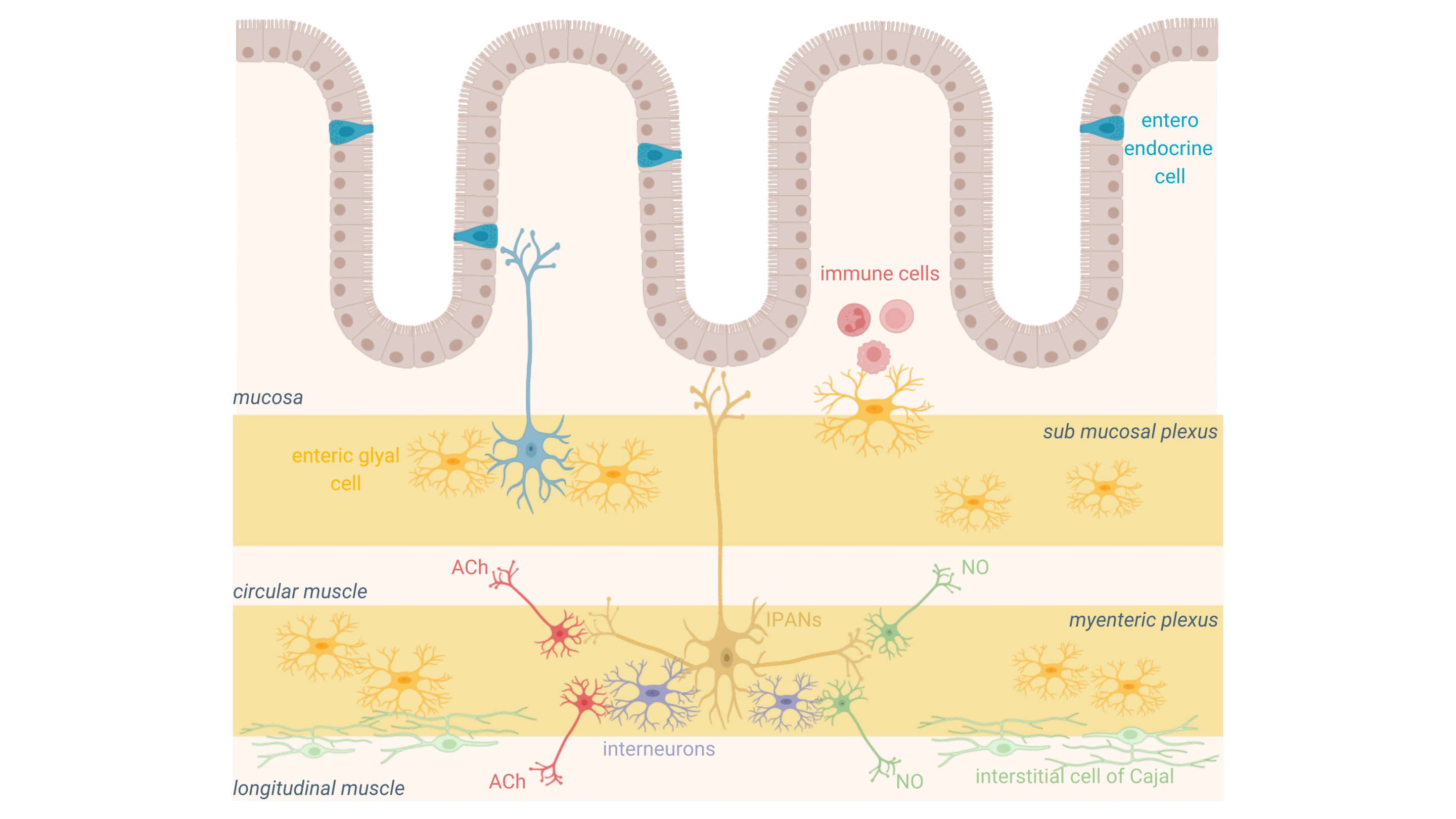
The enteric nervous system at the intestinal level
The enteric nervous system is a complex network of neurons and glial cells embedded in the gastrointestinal tract, structured around the myenteric and submucosal plexi. These networks of digestive neurons regulate key functions such as gut motility, secretions, and nutrient absorption, ensuring the smooth operation of the digestive system.
The systemic role of the enteric nervous system (Gut-Brain axis)
While the ENS operates independently in a short reflex context, it is closely linked to the central nervous system via the gut-brain axis, a bidirectional communication network. This system monitors and regulates digestion, mood, and immune responses, highlighting the ENS’s central role in maintaining systemic health.
How the enteric nervous system communicates with the microbiota and brain?
Microbiota’s role in modulating digestive neurons
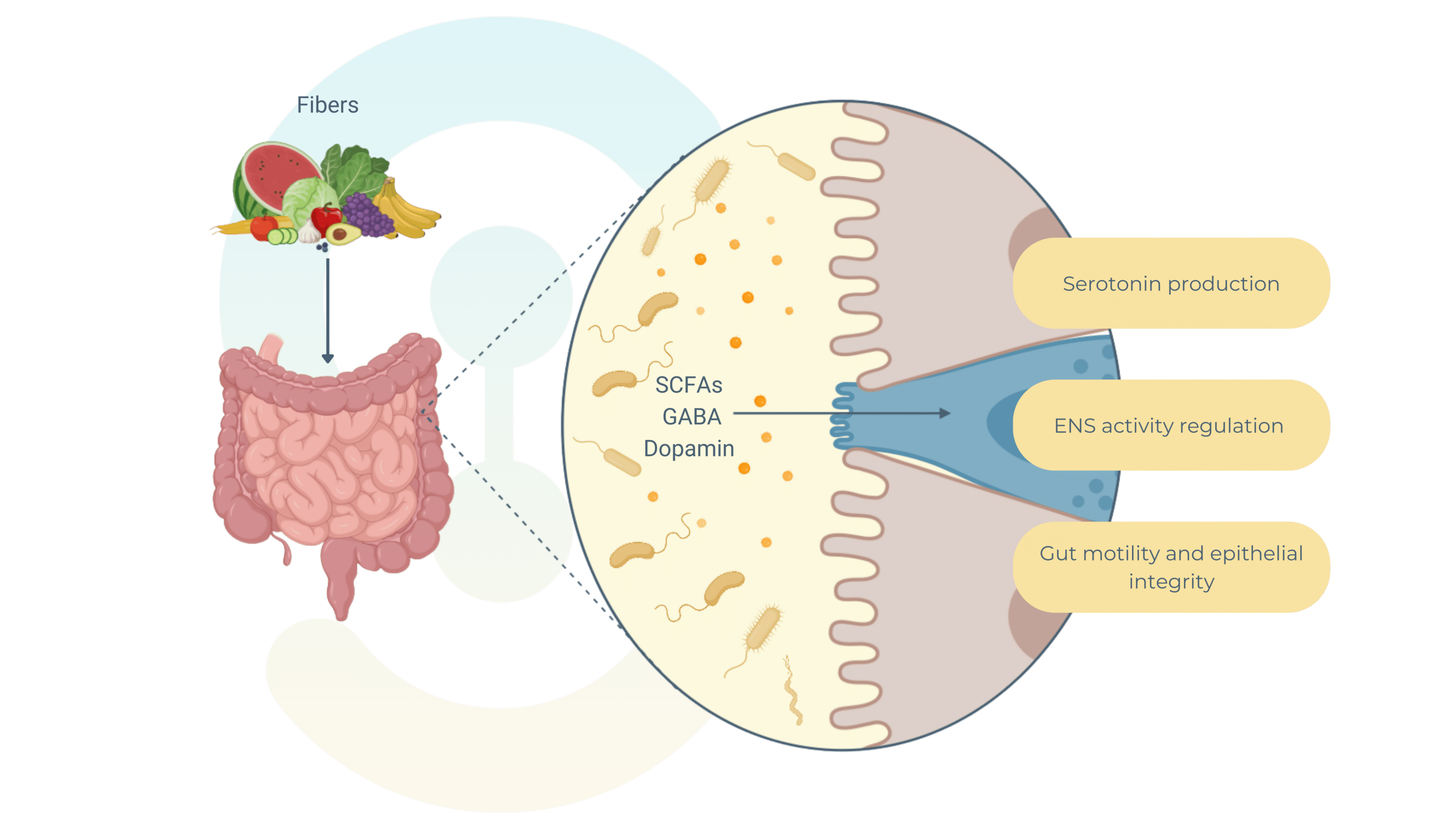
The gut microbiota plays a crucial role in regulating the enteric nervous system by producing metabolites and signaling molecules like short-chain fatty acids (SCFAs)—acetate, propionate, and butyrate—and neurotransmitters such as serotonin. These substances are key mediators of gut-brain communication.
SCFAs, derived from the fermentation of dietary fibers by gut bacteria, interact with specific receptors on enteric neurons to influence gut functions.
For example, acetate impacts gut motility by altering smooth muscle contractility. Butyrate supports epithelial integrity and modulates neuronal excitability, affecting motility and secretion. SCFAs also regulate gut inflammation and immune responses.
The gut produces nearly 90% of the body’s serotonin, largely regulated by microbiota. Bacterial metabolites stimulate enterochromaffin cells to release serotonin, which enhances peristalsis by activating intrinsic afferent neurons and regulates electrolyte secretion and signals discomfort to the central nervous system.
Other microbiota-derived neurotransmitters, like GABA and dopamine, directly influence ENS neurons, shaping stress responses and emotional states, further reinforcing the bidirectional communication between the gut and brain.
Enteric glial cells and their impact on the gut
Enteric glial cells are essential for maintaining intestinal homeostasis and regulating inflammation within the enteric nervous system. These cells support digestive neurons by releasing key factors like glial cell-derived neurotrophic factor (GDNF), which strengthens the epithelial barrier and protects neurons from stress and damage. EGCs also regulate gut motility through the release of nitric oxide (NO) and ATP, ensuring smooth peristalsis and secretion.
In addition, EGCs play a critical role in immune modulation, responding to inflammatory signals by producing cytokines such as IL-6 and TNF-α, which help balance the immune response in the gut. Dysregulation of EGC activity is linked to chronic conditions like inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS). Next month, my newsletter will take a closer look at the enteric nervous system and its essential role in gut homeostasis at the intestinal level !
Depression and the enteric nervous system
Dysfunction in the gut-brain axis has been strongly linked to mood disorders such as depression, with growing evidence highlighting the role of the enteric nervous system and its interactions with the gut microbiota. The ENS communicates with the central nervous system through the vagus nerve, immune signaling, and microbial metabolites, forming a critical pathway for maintaining mental health. When this axis is disrupted, it can lead to neuroinflammation, altered neurotransmitter production, and systemic stress responses, contributing to depressive symptoms.
Chronic stress or dysbiosis can activate the hypothalamic-pituitary-adrenal axis, increasing cortisol levels and driving systemic inflammation. This leads to the release of pro-inflammatory cytokines, such as IL-6 and TNF-α, which can impair ENS signaling and disrupt the blood-brain barrier, exacerbating neuroinflammatory pathways linked to depression.
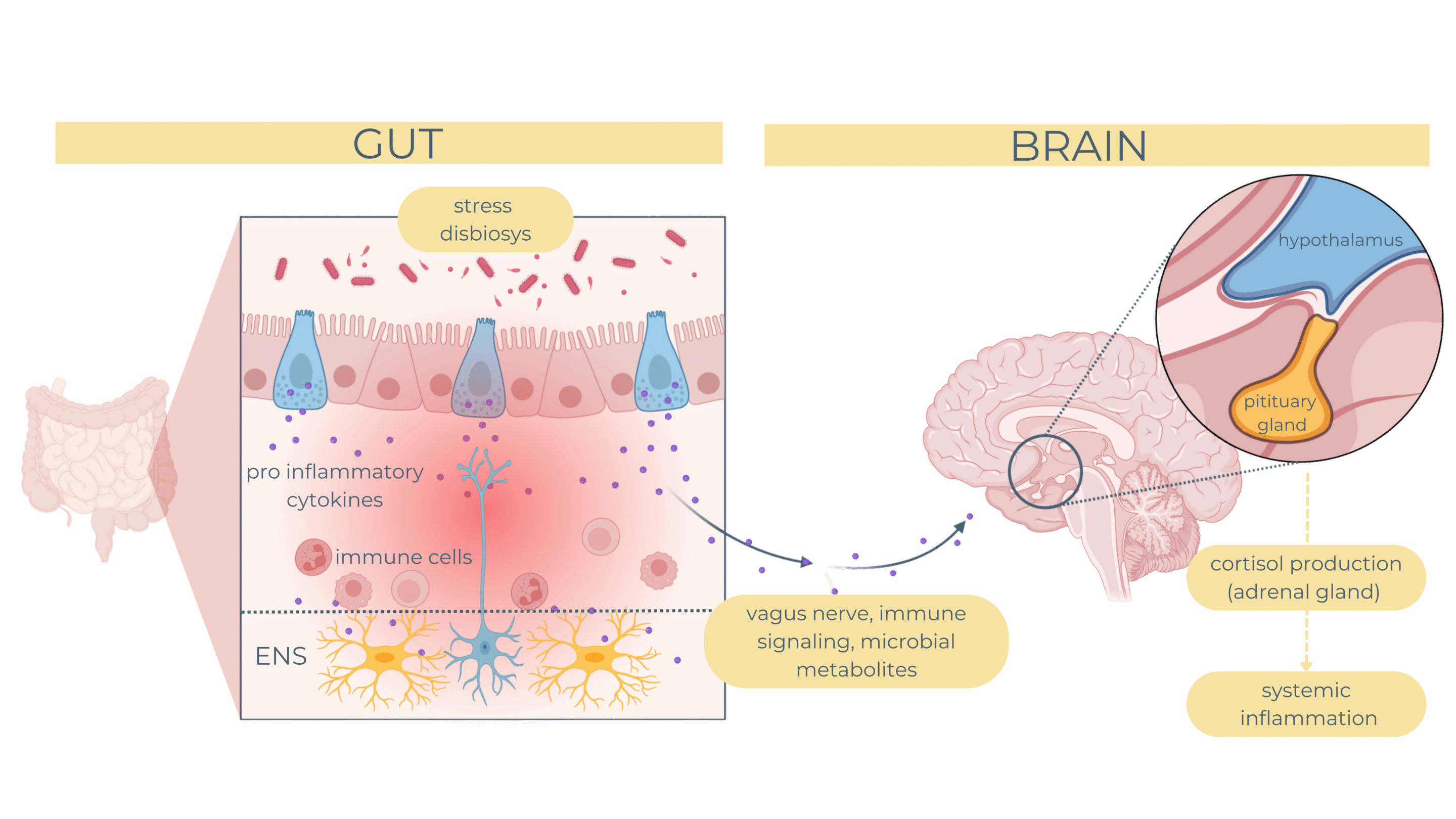
The role pf EGCs extends to the gut-brain axis, where its influence mood and cognition by modulating serotonin release and neuroinflammatory pathways. Reduced GDNF levels in stress-related disorders highlight their potential as therapeutic targets for both gut and mental health conditions.
To restore balance within the gut-brain axis and improve overall health, several approaches are emerging:
- Microbiota modulation (probiotics, prebiotics & postbiotics): specific bacterial strains, such as Lactobacillus and Bifidobacterium, promote serotonin production, reduce inflammation, and enhance stress resilience.
- Nutritional approaches (functional foods): fiber-rich diets stimulate the production of short-chain fatty acids (SCFAs), like butyrate, which reinforce gut barrier integrity and support ENS function.
- Targeted therapies: innovative treatments aim to regulate the microbiota to modulate enteric nervous system activity and mitigate neuroinflammation.
The role of enterosynes in metabolic health
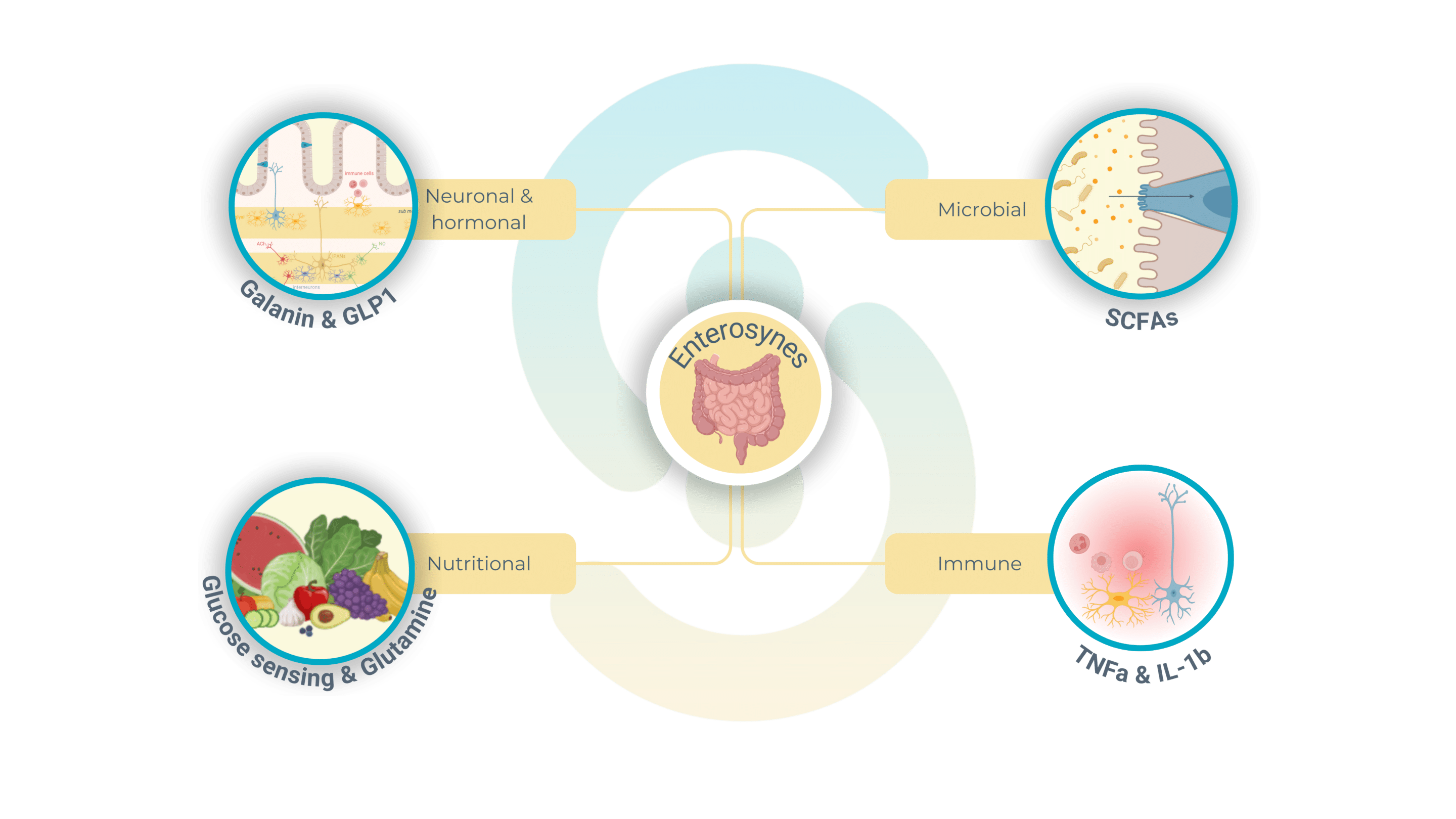
Enterosynes and their connection to digestive neurons
Enterosynes are a class of bioactive molecules produced within the gut, derived from microbiota metabolites, dietary nutrients, or intestinal hormones, that play a crucial role in regulating enteric nervous system function. These molecules interact directly with neurons in the myenteric and submucosal plexi, modulating gut motility, secretion, and metabolic signaling.
Enterosynes exert their effects by acting on specific receptors expressed by enteric neurons, such as G-protein coupled receptors, to modulate neuronal signaling pathways. These molecules regulate the activity of intrinsic primary afferent neurons (IPANs), which play a key role in coordinating peristaltic reflexes and ensuring efficient gut motility, digestion, and nutrient absorption. Additionally, enterosynes enhance the interaction between neurons and glial cells, thereby reinforcing intestinal homeostasis and maintaining the functional integrity of the enteric nervous system.
Enterosynes are derived from various sources within the gut environment:
- Gut microbiota: bioactive compounds such as short-chain fatty acids, serotonin, and neurotransmitter-like molecules produced by gut bacteria directly influence the activity of enteric neurons, shaping gut motility and signaling.
- Dietary nutrients: nutrients like glutamine, glucose, and other amino acids serve as precursors for enterosynes, which help regulate energy balance and intestinal function.
- Intestinal hormones: peptides such as GLP-1 (glucagon-like peptide-1) function as enterosynes by modulating insulin secretion, gut motility, and communication between the gut and brain via the ENS, further highlighting their systemic impact.
Therapeutic potential of enterosynes in metabolic disorders
Metabolic disorders such as type 2 diabetes and obesity are accompanied by intestinal dysmotility, a condition characterized by abnormal gut motility that disrupts the processing of nutrients. This dysmotility contributes to hyperglycemia and insulin resistance by impairing nutrient absorption and altering the communication between the enteric nervous system and the brain. Additionally, the dysregulation of gut motility can affect the secretion of key hormones and disrupt mechanisms controlling food intake, further exacerbating metabolic imbalances.
Addressing insulin resistance and food intake
Enterosynes play a critical role in restoring glucose homeostasis and regulating food intake. For example, short-chain fatty acids derived from gut microbiota stimulate the secretion of GLP-1 that enhances insulin sensitivity and suppresses appetite.
Addressing gut motility
In patients with type 2 diabetes (T2D), disrupted signaling between the ENS and the hypothalamus often results in hyperduodenal motility, which exacerbates persistent hyperglycemia. Enterosynes play a key role in breaking this dysfunctional feedback loop by reducing gut motility, thereby helping to regulate glucose levels, insulin sensibility and control appetite.
For example, butyrate, a microbiota-derived enterosyne, has been shown to alleviate intestinal hypercontractility, a common issue in metabolic syndrome. By normalizing gut motility, butyrate supports balanced glucose and lipid metabolism, reducing the risk of metabolic complications.
Enterosys offers advanced preclinical models to evaluate the effects of interventions on digestive neurons, the intestinal plexus, and the gut-brain axis. Our state-of-the-art techniques enable precise analysis of gut motility, ENS signaling, and the role of enteric glial cells, delivering tailored solutions to address challenges in gut health and metabolic disorders.
Curious about how the enteric nervous system (ENS) plays a role in metabolic disorders and how enterosynes could transform the treatment landscape? Stay tuned for my next newsletter, where I’ll dive deeper into the connection between gut motility, metabolic health, and innovative therapeutic strategies. Don’t miss it!
To craft this newsletter, we drew insights from these three key articles 📚✨
Knauf C, Abot A, Wemelle E, Cani PD. Targeting the Enteric Nervous System to Treat Metabolic Disorders? “Enterosynes” as Therapeutic Gut Factors. Neuroendocrinology. 2020;110(1-2):139-146. doi: 10.1159/000500602. Epub 2019 Jul 2. PMID: 31280267.
Fried S, Wemelle E, Cani PD, Knauf C. Interactions between the microbiota and enteric nervous system during gut-brain disorders. Neuropharmacology. 2021 Oct 1;197:108721. doi: 10.1016/j.neuropharm.2021.108721. Epub 2021 Jul 15. PMID: 34274348.
Liang C, Wei S, Ji Y, Lin J, Jiao W, Li Z, Yan F, Jing X. The role of enteric nervous system and GDNF in depression: Conversation between the brain and the gut. Neurosci Biobehav Rev. 2024 Dec;167:105931. doi: 10.1016/j.neubiorev.2024.105931. Epub 2024 Oct 23. PMID: 39447778.