Understanding the gut-brain axis in type 2 diabetes
Type 2 diabetes is a chronic metabolic disorder characterized by insulin resistance, impaired glucose metabolism, and hyperglycemia. While traditional treatments focus on managing blood glucose levels through pharmacological interventions, emerging research highlights the critical role of the gut-brain axis in regulating glycemia. This intricate communication network between the gut and brain involves sensory receptors, neuronal signals, and metabolic feedback loops that influence glucose metabolism at a systemic level.
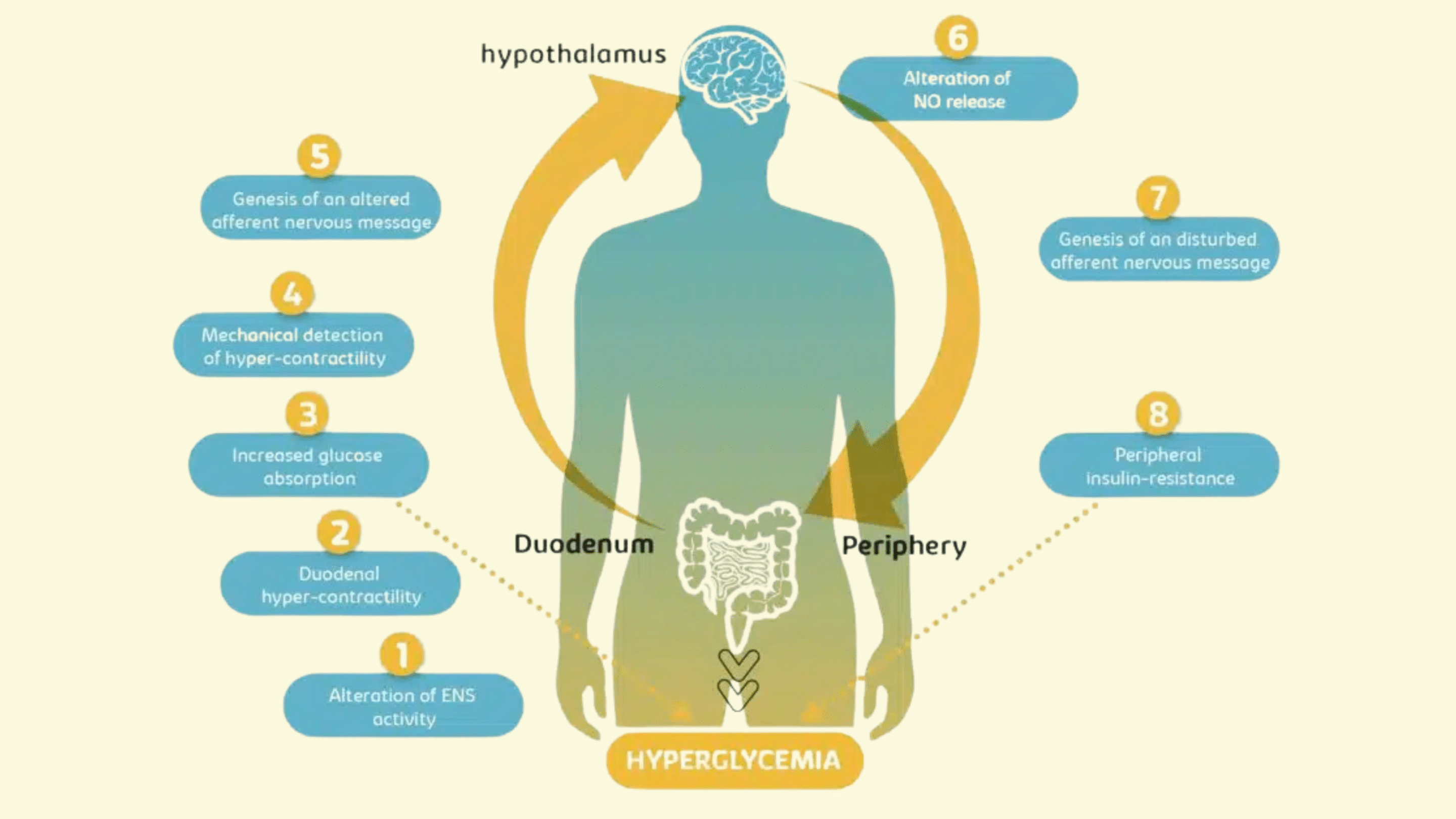
In the intestine, gut distension and nutrient presence are detected by mechanoreceptors and chemoreceptors. These receptors send afferent signals to the hypothalamus, a key brain region involved in metabolic regulation. The hypothalamus, in turn, sends signals to peripheral tissues, such as skeletal muscle, adipose tissue, and the liver, to control glucose uptake and maintain blood glucose levels. When this gut-brain axis communication is disrupted, it can contribute to the progression of this pathology, leading to insulin resistance and postprandial hyperglycemia.
Mechanism of action in type 2 diabetes
One of the key pathological features observed in this pathology is the alteration of enteric nervous system (ENS) activity, which results in duodenal hypercontractility. This excessive contraction of the duodenal smooth muscle contributes to elevated glucose levels during the postprandial period. Additionally, the aberrant nervous signals sent to the hypothalamus disrupt normal metabolic feedback, further exacerbating insulin resistance.
In a healthy gut-brain communication system, the hypothalamus receives accurate neural signals from the ENS, allowing it to regulate glucose uptake effectively. However, in type 2 diabetes, this pathway is disrupted, causing impaired hypothalamic nitric oxide (NO) signaling. Nitric oxide is essential for hypothalamic neurons to send appropriate regulatory signals to peripheral tissues. Without proper NO signaling, tissues such as skeletal muscle, liver, and adipose tissue fail to respond adequately to insulin, leading to poor glucose uptake and persistent hyperglycemia.
This breakdown in the gut-brain axis highlights a critical therapeutic target: reducing duodenal hypercontractility to restore the gut-brain communication pathway and improve overall glucose metabolism.
Targeting mechanosensing: a novel therapeutic approach
Future therapeutic strategies for type 2 diabetes are increasingly focusing on targeting gut mechanoreceptors and the enteric nervous system to modulate duodenal contractions. The objective is clear: restore proper gut-brain signaling and prevent insulin resistance from worsening.
Research led by Professor Claude Knauf has uncovered bioactive peptides, including apelin, galanin, and enkephalin, which play a significant role in reducing duodenal hypercontractility. Apelin, released by enterocytes, and galanin and enkephalin, secreted by enteric neurons, have shown promising effects in stimulating neurons within the myenteric plexus. These neurons are responsible for relaxing smooth muscle cells in the duodenum, thereby reducing hypercontractility and improving gut motility.
In preclinical studies, these bioactive peptides were shown to restore hypothalamic activity in diabetic mice. Once duodenal contractions returned to normal, hypothalamic NO production increased, enabling the hypothalamus to send appropriate regulatory signals to peripheral tissues. As a result, glucose sensitivity in skeletal muscle, liver, and adipose tissue improved, and hyperglycemia was significantly reduced.
These findings suggest that targeting gut-brain axis communication via bioactive peptides could become a cornerstone in future type 2 diabetes treatments. Such therapies offer the potential to address the root cause of insulin resistance rather than merely managing blood glucose levels.
The Role of gut microbiota in this pathology
The gut microbiota is emerging as a key player in regulating gut-brain axis communication and, by extension, glucose metabolism. Researchers have shown that specific gut bacteria can influence the production of bioactive peptides like apelin, galanin, and enkephalin, which are crucial for regulating ENS activity.
In type 2 diabetes, gut dysbiosis—a disruption in the balance of gut bacteria—can impair the production and release of these bioactive peptides. This disruption further exacerbates duodenal hypercontractility, weakens gut-brain signaling, and worsens insulin resistance.
Nutritional approaches, including prebiotic and probiotic supplementation, are now being explored as complementary strategies alongside pharmacological treatments. By modulating the gut microbiota, it may be possible to restore the production of essential bioactive peptides, improve gut-brain axis communication, and support overall metabolic health.
This dual approach—combining targeted pharmacological interventions with nutritional strategies—offers a more holistic framework for treating type 2 diabetes and preventing disease progression.
Partner with enterosys for advanced research on type 2 diabetes
At Enterosys, we are committed to advancing the understanding of the gut-brain axis and its role in metabolic diseases like type 2 diabetes. Our expertise in gut-organ interactions allows us to deliver innovative insights and preclinical in vitro and in vivo models that pave the way for next-generation therapies!